Abstract
Introduction: The preferred post-remission therapy for older patients (pts) with AML remains uncertain. We compared outcomes for older AML pts in CR1 receiving HCT reported to the CIBMTR to older AML pts achieving CR1 on National Clinical Trials Network induction and non-HCT consolidation therapy (CT) trials.
Methods: This study focused on pts 60-75 years of age treated between 2004 to 2013. CT pts (n=211) underwent induction and consolidation on Alliance for Clinical Trials in Oncology, ECOG-ACRIN or SWOG clinical trials for initial therapy for newly diagnosed AML; the CIBMTR provided data for HCT pts (n=431). CT patients received at least one cycle of CT on study and were excluded if HCT occurred at any time. Time to event started at CR1 and pts entered at CT or HCT, respectively, using left-truncation to account for differential entry times.
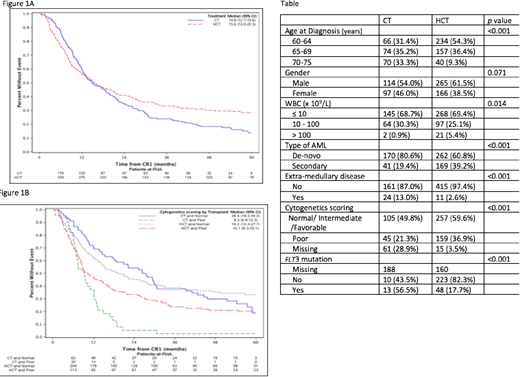
Results: For the CT cohort, first consolidation included standard therapy (e.g., cytarabine or a hypomethylating agent) and additional study drug (e.g., bortezomib, dasatinib, sorafenib, and Zosuquidar, gemtuzumab) or tipifarnib alone. Among HCT pts, the donor was a HLA-matched sibling or unrelated donor (URD) in 66% and the others were partially HLA-matched/mismatched URD (10%) or cord blood (24%). HCT pts were younger and more frequently had high-risk AML (high WBC, secondary AML and unfavorable cytogenetics) (Table). The median time from CR1 to HCT and CT was 3.2 and 0.5 months, respectively.
Allogeneic HCT showed worse overall survival (OS) (HR=1.52, p=0.02) prior to 9 months and better OS thereafter (HR= 0.53, p <0.0001) relative to CT (figure 1A). Treatment-related mortality (TRM) was worse after HCT in the first 9 months (HR=2.8, CI: 1.5 -5.2, p=.0009), while relapse was less frequent beyond 9 months after treatment (HR = 0.42, CI: 0.29 to 0.61, p<.0001). Despite higher early TRM, HCT recipients went on to manifest superior OS [5 year OS: HCT 29% (24-34%), CT 13.8% (9 -22%)] (Figure 1A). The benefit of HCT for survival after 9 months was more prominent in those with unfavorable cytogenetics (Figure 1B). Multivariate analysis for OS showed no statistically significant effect of age or performance status, while unfavorable cytogenetics were detrimental (HR=1.74, p<.0001).
Conclusions. Allogeneic HCT led to heightened early risks from TRM, but resulted in superior long-term survival in older AML pts receiving HCT relative to CT by reducing relapse. Efforts to attenuate early TRM after allogeneic HCT may further improve HCT outcomes for older pts.
Ustun:novartis: Speakers Bureau. Attar:Agios: Employment, Equity Ownership. Larson:Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; BristolMyers Squibb: Consultancy, Research Funding; Ariad/Takeda: Consultancy, Research Funding. Roboz:Roche/Genentech: Consultancy; Eisai: Consultancy; Cellectis: Research Funding; Aphivena Therapeutics: Consultancy; Sandoz: Consultancy; Janssen Pharmaceuticals: Consultancy; Otsuka: Consultancy; Jazz Pharmaceuticals: Consultancy; Sandoz: Consultancy; AbbVie: Consultancy; Celltrion: Consultancy; Cellectis: Research Funding; Eisai: Consultancy; Astex Pharmaceuticals: Consultancy; Argenx: Consultancy; Pfizer: Consultancy; Celgene Corporation: Consultancy; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy; Bayer: Consultancy; Bayer: Consultancy; Roche/Genentech: Consultancy; Orsenix: Consultancy; Astex Pharmaceuticals: Consultancy; Celgene Corporation: Consultancy; Daiichi Sankyo: Consultancy; Pfizer: Consultancy; Janssen Pharmaceuticals: Consultancy; Otsuka: Consultancy; Orsenix: Consultancy; Daiichi Sankyo: Consultancy; Celltrion: Consultancy; Novartis: Consultancy; Aphivena Therapeutics: Consultancy; Argenx: Consultancy; AbbVie: Consultancy. Uy:GlycoMimetics: Consultancy; Curis: Consultancy. Stone:Fujifilm: Consultancy; Ono: Consultancy; Orsenix: Consultancy; Otsuka: Consultancy; Agios: Consultancy, Research Funding; AbbVie: Consultancy; Argenx: Other: Data and Safety Monitoring Board; Celgene: Consultancy, Other: Data and Safety Monitoring Board, Steering Committee; Arog: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Jazz: Consultancy; Astellas: Consultancy; Merck: Consultancy; Pfizer: Consultancy; Sumitomo: Consultancy; Amgen: Consultancy; Cornerstone: Consultancy. Foran:Agios: Research Funding; Xencor, Inc.: Research Funding. Weisdorf:Seattle Genetics: Consultancy; Equillium: Consultancy; FATE: Consultancy; SL Behring: Consultancy; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal